From Rice University, Houston … Bringing order to disorder is key to making stronger and greener cement and concrete. In National Science Foundation and U.S. Department of Energy-backed research, Rice University scientists have decoded the kinetic properties of cement and developed a way to “program” the microscopic, semicrystalline particles within. The process turns particles from disordered clumps into regimented cubes, or spheres that combine to make the material less porous and more durable.
Their study appears in the Royal Society of Chemistry’s Journal of Materials Chemistry. The technique it describes may lead to stronger structures that require less concrete. Less is better, says lead author and Rice University Materials Scientist Rouzbeh Shahsavari, who cites the 3 billion-plus tons of carbon dioxide released into the atmosphere from worldwide portland cement production.
Through extensive experiments, he and colleagues decoded nanoscale reactions, or “morphogenesis,” of the crystallization within calcium-silicate hydrate (C-S-H), the hydrated-cement compound binding aggregates into concrete. For the first time, they synthesized C-S-H particles in a variety of shapes, including cubes, rectangular prisms, dendrites, core-shells and rhombohedra, then mapped them into a unified morphology diagram for producers and practitioners who wish to engineer concrete from the bottom up.
“We call it programmable cement,” explains Shahsavari. “The great advance of this work is that it’s the first step in controlling the kinetics of cement to get desired shapes. We show how one can control the morphology and size of the basic building blocks of C-S-H so that they can self-assemble into microstructures with far greater packing density compared with conventional amorphous C-S-H microstructures.”
The idea is akin to the self-assembly of metallic crystals and polymers. “It’s a hot area and researchers are taking advantage of it,” Shahsavari notes. “But when it comes to cement and concrete, it is extremely difficult to control their bottom-up assembly. Our work provides the first recipe for such advanced synthesis.
“The seed particles form first, automatically, in our reactions, and then they dominate the process as the rest of the material forms around them. That’s the beauty of it. It’s in situ, seed-mediated growth and does not require external addition of seed particles, as commonly done in the industry to promote crystallization and growth.”
Previous techniques to create ordered crystals in C-S-H required high temperatures or pressures, prolonged reaction times and the use of organic precursors, but none were efficient or environmentally benign, Shahsavari observes. The Rice University lab created well-shaped cubes and rectangles by adding small amounts of positive or negative ionic surfactants and calcium silicate to C-S-H, and exposing the mix to carbon dioxide and ultrasonic sound. The crystal seeds took shape around surfactant micelles within 25 minutes. Decreasing the calcium silicate yielded more spherical particles and smaller cubes; increasing it formed clumped spheres and interlocking cubes.
|
|
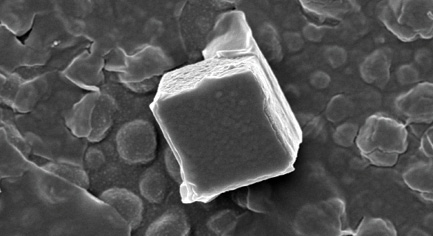
| Work in the Rice University lab netted an isolated cement cube. Microscopic cubes and other shapes may serve as “seeds” in programmable cement for stronger, less porous and more environmentally friendly concrete. PHOTO: Rice University Multiscale Materials Laboratory |
Once the calcite “seeds” form, they trigger the molecules around them to self-assemble into cubes, spheres and other shapes that are orders of magnitude larger. These can pack more tightly together in concrete than amorphous particles. Carefully modulating the precursor concentration, temperature and duration of the reaction varies the yield, size and morphology of the final particles.
The discovery is an important step in concrete research, building upon work of the Massachusetts Institute of Technology team that decoded cement’s molecular “DNA” in 2009, Shahsavari affirms. “There is currently no control over C-S-H shape,” he adds. “The concrete used today is an amorphous colloid with significant porosity that entails reduced strength and durability.”
Concrete is one focus of the Rice lab, which has studied both its macroscale manufacture and intrinsic nanoscale properties. Because concrete is the world’s most common construction material and a source of atmospheric carbon dioxide, university staff are convinced of the importance of developing a “greener” matrix.
 |
| Rice University materials scientists have mapped the morphogenesis of cement hydrates used in concrete. Their work could lead to finer control of the microscopic shape of cement particles for the manufacture of stronger, more durable concrete. ILLUSTRATION: Rice University Multiscale Materials Laboratory |
The new technique has several environmental benefits, says Shahsavari: “One is that you need less of it (the concrete) because it is stronger. This stems from better packing of the cubic particles, which leads to stronger microstructures. The other is that it will be more durable. Less porosity makes it harder for unwanted chemicals to find a path through the concrete, so it does a better job of protecting steel reinforcement inside.”
The research required the team to develop a method to test microscopic concrete particles for strength. Researchers used a diamond-tipped nanoindenter to crush single cement particles with a flat edge. They programmed the indenter to move from one nanoparticle to the next and crush it and gathered mechanical data on hundreds of particles of various shapes in one run.
“Other research groups have tested bulk cement and concrete, but [none] had ever probed the mechanics of single C-S-H particles and the effect of shape on mechanics of individual particles,” says Shahsavari. Strategies developed during the project could have implications for other applications, he adds, including bone tissue engineering, drug delivery and refractory materials, and could impact such other complex systems as ceramics and colloids.
Joining Rouzbeh Shahsavari on the research team and as Journal of Materials Chemistry paper co-authors are Professor of Chemisty Kenton Whitmire; Rice postdoctoral researcher Sakineh Moghadda; graduate students Sung Hoon Hwang and Shuo Zhao; postdoctoral researcher Sreeprasad Srinavasan; undergraduate Benhang Shi; Aali Alizadeh, Vahid Hejazi and Joseph Miller of C-Crete Technologies in Houston; and, Irene Rusakova, a senior research scientist at the University of Houston.
CONCRETE SUSTAINABILITY HUB MODEL THERMAL CRACKS IN PAVEMENTS
 |
| CSHub researchers calculated cracking risk over time following a temperature change. The process is time-dependent because heat takes time to diffuse. Each line represents a pavement with a different thermal diffusion coefficient (a measure of how effectively the pavement is able to conduct heat). The temperature profile is expected to eventually be the same for all systems (represented by the dashed line), but intermediate values differ due to the difference in heat transfer. |