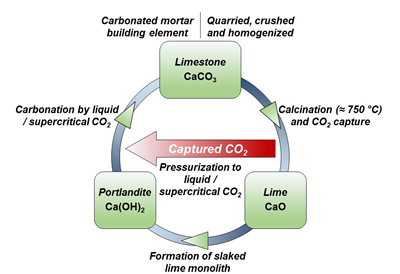
Targeting lower net carbon dioxide emissions in portland cement milling, University of California, Los Angeles researchers propose introduction of calcium hydroxide at the calcination phase, when high temperatures split limestone raw feed into CO2 gas and calcium oxide. Lab-scale tests in their National Science Foundation-backed investigation indicate the potential to sequester CO2 and recreate limestone in a continuous loop.
Writing in the American Chemical Society’s Industrial & Engineering Chemistry Research journal, UCLA Associate Professor of Civil and Environmental Engineering Gaurav Sant and “Direct Carbonation of Ca(OH)2 Using Liquid and Supercritical CO2: Implications for Carbon-Neutral Cementation” co-authors examine calcination, the principal source (up to 65 percent) of CO2 emissions in portland cement milling, plus a secondary source (up to 35 percent): the fuel combustion behind heating lime and siliceous sand to form tricalcium silicate. The processing cycle they envision would reduce by about 50 percent the heat required for tricalcium silicate, compounding CO2 emissions reduction realized in alternative calcination.
Professor Sant notes that the latter process is analogous to marine life mineral binding, where limestone is formed through microbe activity, in turn cementing sand grains together for shells or exoskeletons. Scientists previously examined such limestone formation, he adds, but had not demonstrated it with a view to carbon dioxide-neutral cement production. Now that the process has been proved in a lab environment, it could in time be scaled to commercial levels.
The CO2-sequestering and emissions reduction study aligns with the UCLA Sustainable LA Grand Challenge, aiming to transition the Los Angeles region to 100 percent renewable energy local water and enhanced ecosystem health by 2050. UCLA peers joining Professor Sant in the cement research as “Direct Carbonation” co-authors are Assistant Professor of Civil and Environmental Engineering Mathieu Bauchy; research scientist Magdalena Balonis; postdoctoral scholars Kirk Vance and Isabella Pignatelli; and, doctoral scholar Gabriel Falzone. Their investigation was conducted across UCLA facilities: Laboratory for the Chemistry of Construction Materials in the Henry Samueli School of Engineering and Applied Science; Electron Imaging Center for Nanomachines at the California NanoSystems Institute; and, Department of Chemistry and Biochemistry Molecular Instrumentation Center.
CEMENT PLANTS SEE ENERGY-EFFICIENT YEAR
Portland Cement Association’s new U.S. Labor-Energy Input Survey shows the amount of energy required to produce one ton of portland cement—4.4 million Btu, on average—dropped 1.1 percent in 2014 from previous levels, and equates to domestic plants’ most energy efficient year on record. The survey also finds rising use of alternative fuels, which provided 15 percent of total plant energy needs in 2014 and are now part of the energy mix in 75 percent of U.S. cement mills.