Borrowing from a “rigidity theory” that led Corning Inc. engineers to the high-performance Gorilla Glass, the Massachusetts Institute of Technology-hosted Concrete Sustainability Hub is studying potential portland cement reformulation aimed at higher fracture resistance than current ASTM C 150 product.
 |
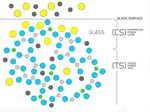
Corning’s sample diagram of aluminum (green), oxygen (blue) and silicon (gray) atoms, plus potassium (yellow) and sodium (charcoal) ions, shows the process imparting robust Gorilla Glass surface characteristics: In a molten-salt bath at 400°C, potassium ions replace sodium ions near the surface. |
“Tougher cement would allow using less material while achieving comparable mechanical properties. Increased resistance to fracture would improve cement’s longevity, making it even more sustainable. As such, it is of primary importance to understand how composition affects the resistance to fracture of calcium-silicate-hydrates, starting from the atomic scale,” CSHub notes in its “Gorilla cement: Tougher, yet greener” Research Brief.
Fracture properties and rigidity can hinge on volume adjustments of three common salts in conjunction with the pyroprocessing behind portland cement and glass. Gorilla Glass production requires replacement of small sodium ions with larger potassium ions in the molten phase, imparting high compressive stress deep into the final product.
In the portland cement parallel, CSHub researchers are focusing on clinker phase chemistry changes netting higher silica content in calcium-silicate-hydrate (C-S-H), the principal binder in concrete. They aim to optimize the atomic structure of C-S-H, which at the nanoscale has connected calcium, silicon, oxygen, and hydrogen atoms in a steel truss-like network.
“The atomic structure of C-S-H can be optimized by changing the ratio of joints, or atoms, versus sticks, or the chemical constraints acting between the atoms,” explains University of California, Los Angeles Assistant Professor Mathieu Bauchy, Ph.D., who is visiting the MIT Department of Civil and Environmental Engineering and assisting in CSHub nanoscience applications. “In practice, this is achieved by modifying the chemistry of C-S-H, which is mainly captured by the proportion of calcium versus silicon atoms, hence the Ca/Si molar ratio.”
 |
Targeting cement clinker modifications toward increased slab and structure performance, Concrete Sustainability Hub researchers pinpoint an optimized ratio of calcium: silicon atoms in calcium-silicate-hydrate. Attainment of a 1.5 Ca/Si molar ratio in C-S-H grains could replicate in finished concrete the elevated toughness and fracture resistance Corning Inc. chemists and engineers have realized in Gorilla Glass. The grains’ fracture toughness is optimal for isostatic compositions, measured when the Ca/Si molar ratio hovers 1.5, while MIT researchers characterize flexible and stressed-rigid compositions, respectively, at 1.7 and 1.3 ratios—the former most representative of typical portland cement. |
“When the number of chemical constraints per atom is too low, the material is flexible, similar to a deformable mechanical truss. In that case, the material would lose its cohesiveness,” he adds. “When the number of constraints per atom is too high, the material cannot deform at all and becomes brittle. In between, we identify an optimal state, named isostatic or statically determined, for which the material is the toughest, having at the same time high cohesiveness and the ability to relax energy by deforming under stress. This is what we call ‘Gorilla cement,’ characterized by a Ca/Si molar ratio of 1.5.”
“Gorilla Glass reduces complex molecular networks to simple mechanical trusses,” note Dr. Bauchy and colleagues in the research brief. “A network can be flexible, stressed-rigid, or isostatic, if, respectively, the number of chemical constraints is lower, higher, or equal to the number of degrees of freedom of the atoms. Isostatic materials are able to deform and feature relatively high surface energy … We predict that decreasing the Ca/Si of C-S-H from 1.7 to 1.5 would increase its toughness by 70 percent, thus allowing use of less material without compromising performance. Decreasing the relative amount of calcium in cement can be achieved by replacing clinker with silica-rich byproduct materials such as fly ash. Tougher and greener, this ‘Gorilla cement’ would help improve the sustainability of our built environment.”
The research continues under Concrete Science, which along with Buildings and Pavements, encompasses areas the Ready Mixed Concrete Research and Education Foundation and Portland Cement Association prioritize as CSHub sponsors.